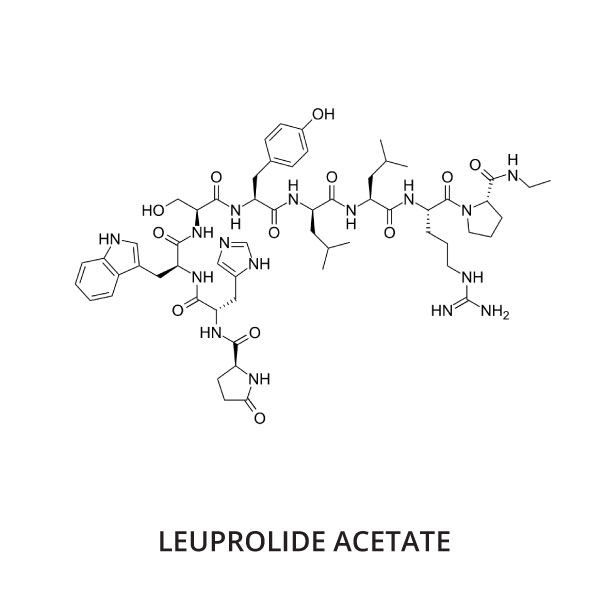
Leuprolide acetate (brand name Lupron®) is a synthetic gonadotropin-releasing hormone (GnRH) which is administered as a series of intramuscular (IM) injections. It is used 'off-label' for the treatment and prevention of a variety of reproductive tract-associated disorders in pet poultry.